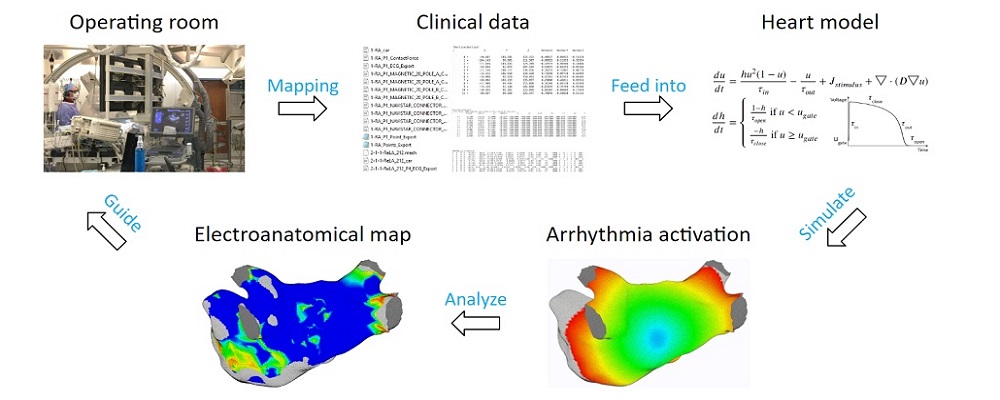
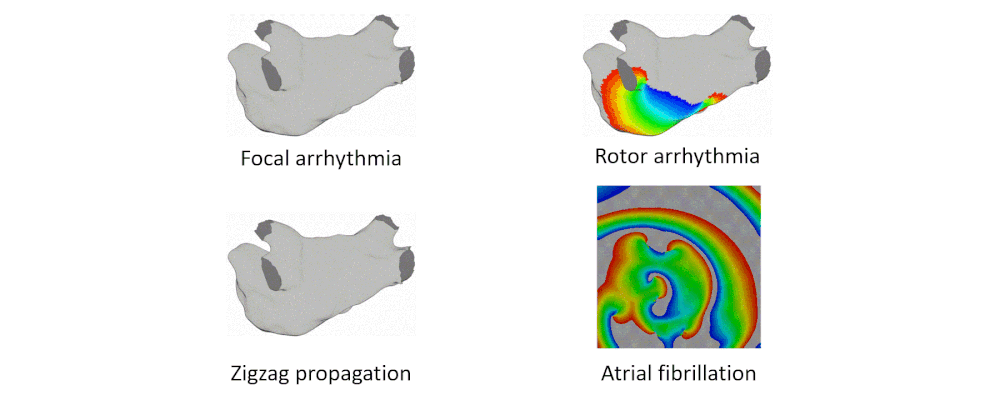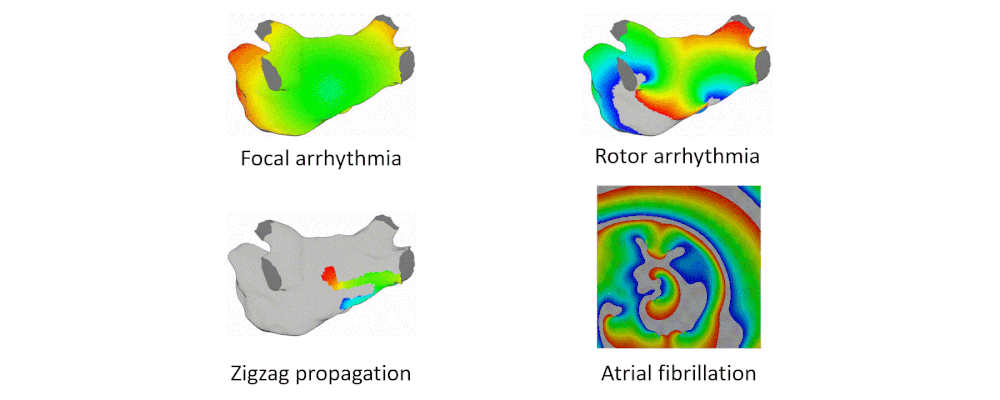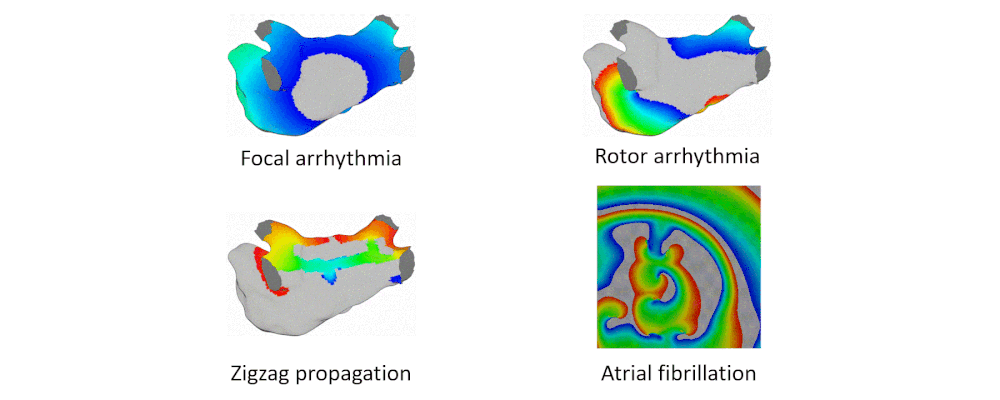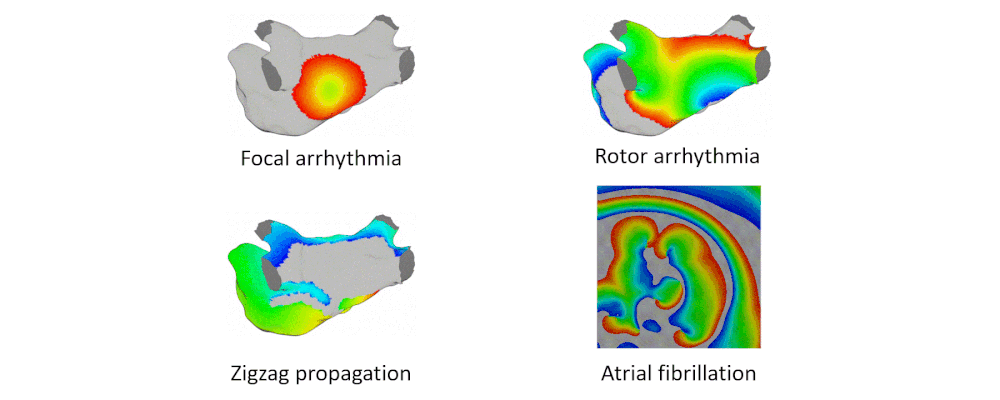
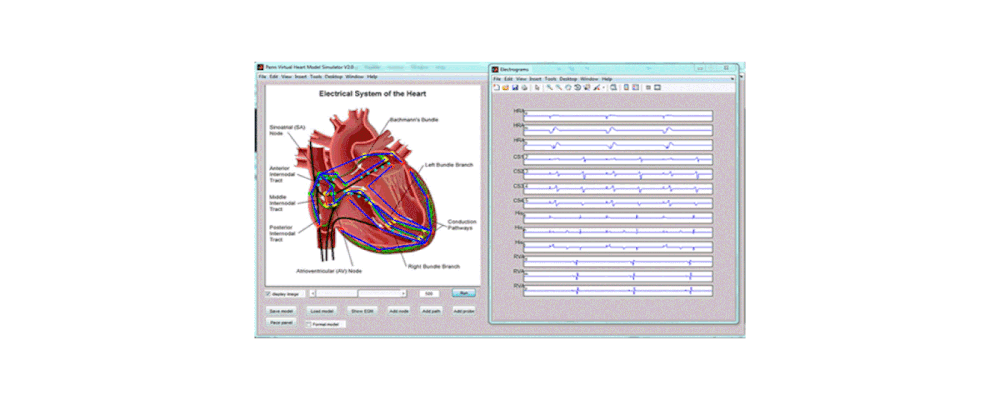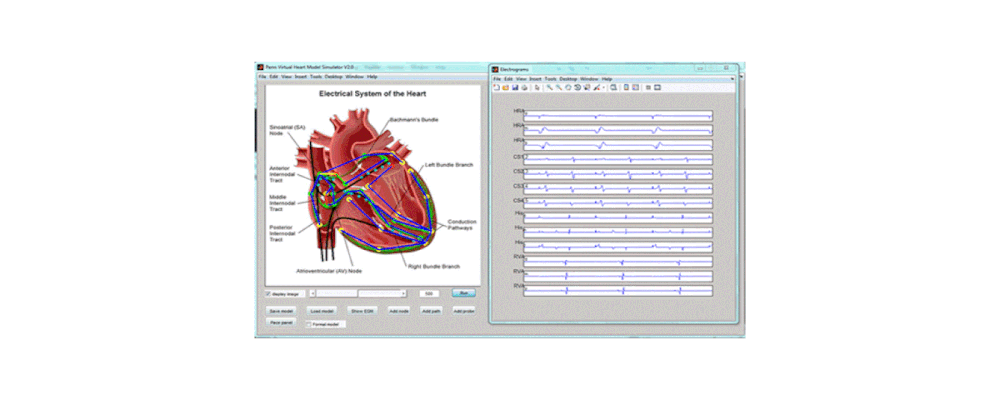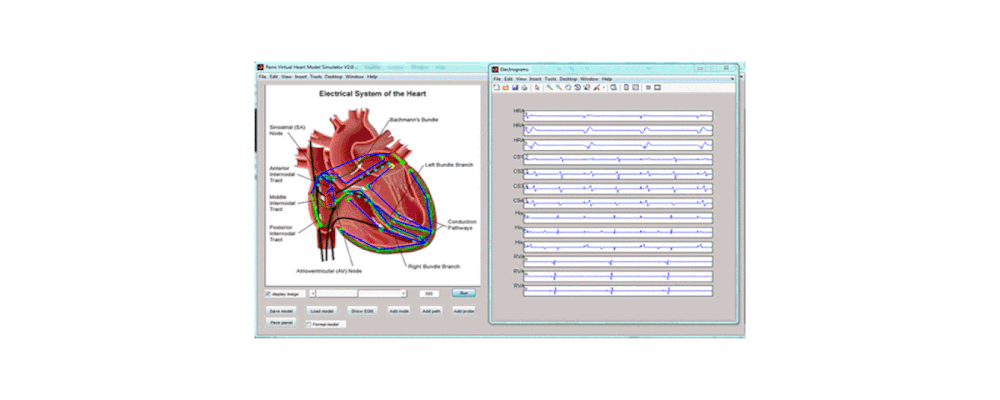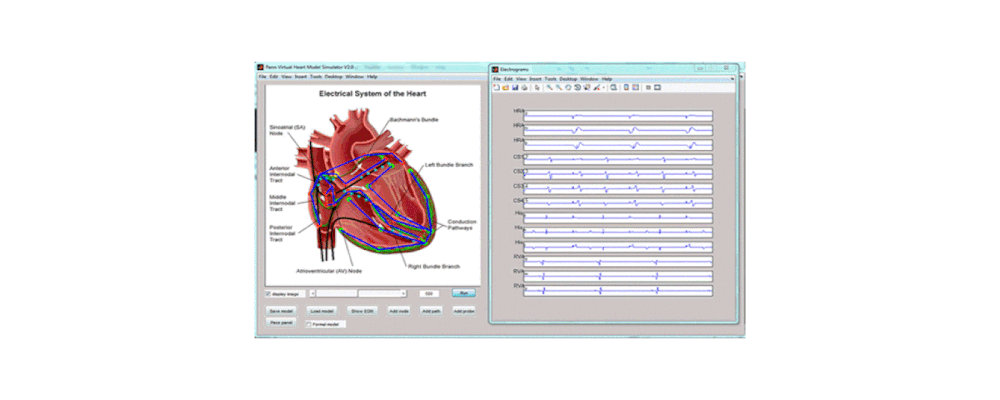
Patient-specific electrophysiological heart model for assisting left atrium arrhythmia ablation
Atrial arrhythmia can be categorized into tachycardia, flutter, and fibrillation. Atrial fibrillation is a prevalent heart disease that results in weak and irregular contractions of the atria. It affects millions people worldwide and contributes to hundreds of thousands deaths annually. Cardiac ablation is among the most successful treatment options, involving the use of radio frequency energy to kill diseased cells or create lesion lines that obstruct abnormal activation waves. During the procedure, catheters are inserted into the left atrium to map the atrium geometry and record endocardium electrograms that are then converted into electroanatomical maps to pinpoint the arrhythmia source locations.
However, identifying these sources is challenging. The electrograms are asynchronous and can be susceptible to noise. The spatial distribution of sampling sites is non-uniform, which leads to inaccurate maps. Identifying arrhythmia source locations is not a trivial task. Therefore, an ablation procedure often lasts from 3 to 6 hours, and arrhythmia recurrence within 12 months after first ablation is around 50%.
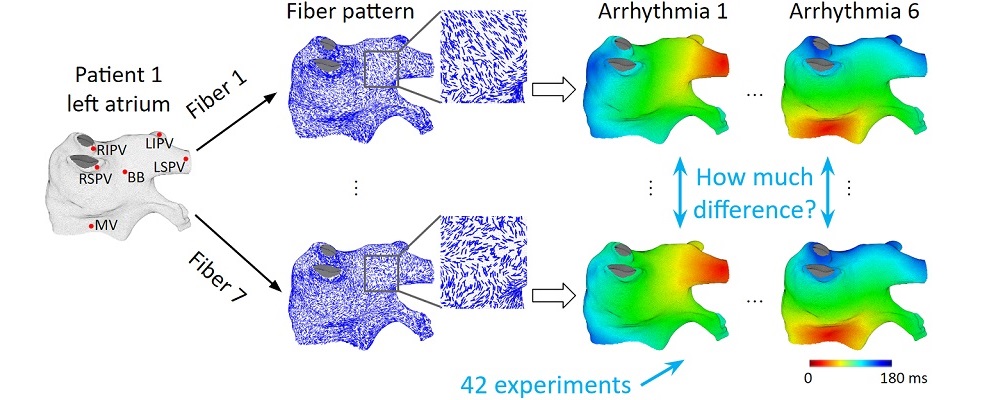
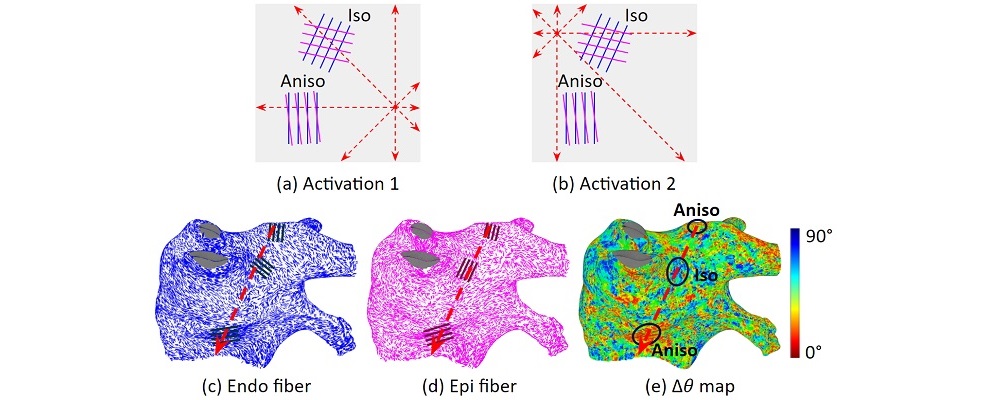
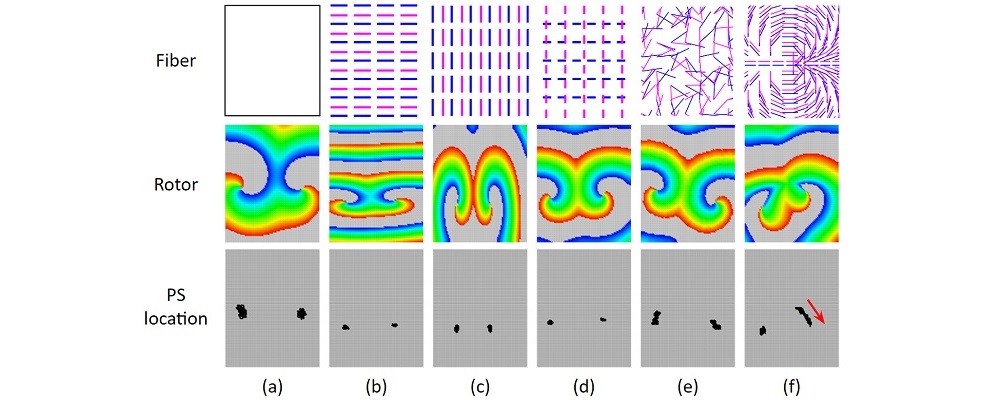
To address these challenges, we developed an integrated computational heart mode for clinical left atrium arrhythmia ablation. Our system takes in the left atrium geometry and electrograms, processes them to extract regional tissue properties, which are used to tune a heart model, creating a patient-specific whole-atrium model. With this model, we can simulate and detect arrhythmia sources, and provide ablation assistance. To build such a system, we investigated the fiber effects on atrial activation patterns. We developed a fast heart model tuning method which takes only a few seconds of computation time on a personal computer, enabling real-time assistance during the ablation procedure. We achieved high accuracy in simulating arrhythmias, which we validated on patient data.